Description
Swiss Point of Care Rapid Flow Swab Test Kits – Pack of 25 Kits
Swiss Point of Care Covid rapid antigen tests are easy to perform and they give accurate information on whether someone is infected with the COVID-19 virus. Because of the ease of using the nasal swab, and the accuracy of the results obtained quickly, this test kit is suitable to be used for testing more people at the same time.
For individual person testing and ideal to quickly test larger groups of people at a very reasonable cost. Regularly testing all your employees within a company or a particular location, warehouse or barracks to halt the spread of infection.
The nasal swab method compares favourably to nasopharyngeal lateral flow tests where the swab needs to be inserted to the back of the throat which is not as comfortable, making these nasal-only kits particularly good for testing children.
Visit our test kit website to learn more about these low cost rapid COVID test kits or see the full range of lateral flow tests for Coronavirus.
Please see instructions within the kit to be followed carefully and also below ‘How-To-Use’ illustration:
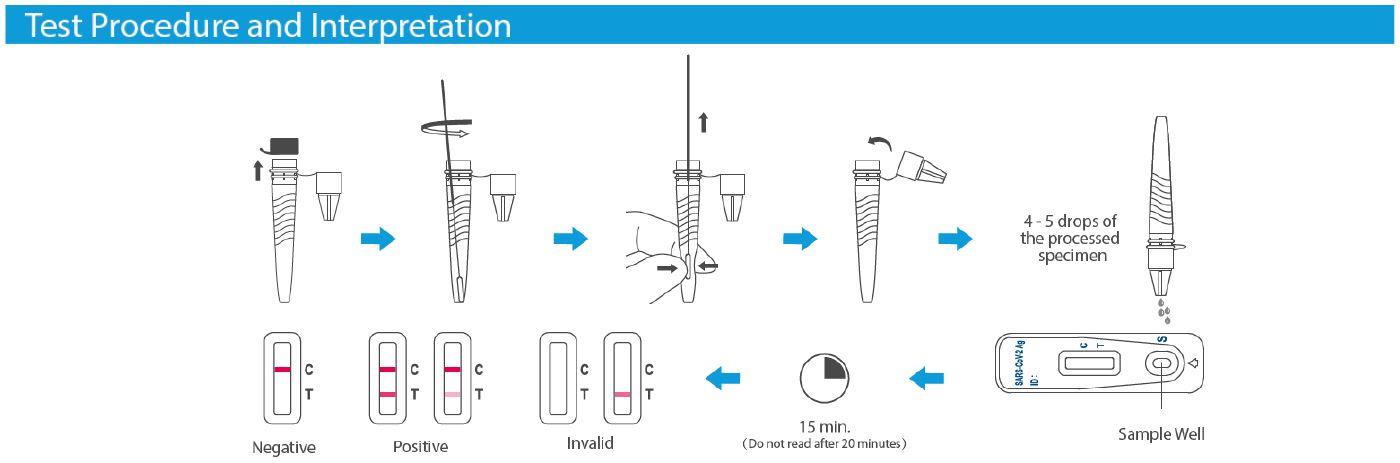
Pack Contents:
25 x complete Covid rapid test kits per box.
Each test includes: 1 rapid test device, 1 swab kit, 1 liquid buffer and 1 pipette.
Accreditations:
Manufactured by Acon Labs in China – Registered by MedNet Gmbh, Germany – authorised representative in accordance with the directive 98/79EC of the European Parliament and the Council of the European Union relating to in vitro diagnostic medical devices.
Legal text of the 98/79EC directive and harmonised standards can be seen here.


Reviews
There are no reviews yet.